Our Company
NexHealth Synergy LLC is a global partner for regulatory compliance, nanotechnology-based R&D, and healthcare digital transformation. We enable medical device and pharmaceutical companies to achieve faster approvals, smarter workflows, and scalable innovation through expert consulting and advanced technology solutions.
At a Glance
NexHealth Synergy LLC is a US-registered global healthcare consulting and innovation company specializing in medical device, pharmaceutical, and healthcare compliance across major international markets. We provide end-to-end regulatory, R&D, and digital transformation services, supporting organizations in achieving faster approvals, efficient operations, and sustainable global expansion. Our expertise spans EU MDR, FDA, CDSCO, and other international frameworks, combined with advanced nanotechnology research and AI-driven SaaS solutions.
NexHealth Synergy LLC, a US-based global healthcare consulting and innovation company, is committed to enabling medical device, pharmaceutical, and healthcare organizations to successfully navigate complex international regulatory landscapes while driving innovation and operational excellence. Our multidisciplinary team brings deep expertise in global medical device and pharmaceutical regulations, combined with advanced research capabilities in nanotechnology and healthcare product development. From early-stage regulatory strategy and technical documentation to submission, compliance management, and post-market support, we deliver comprehensive, end-to-end solutions tailored to the needs of global manufacturers, startups, and healthcare providers.
In addition, we leverage modern digital technologies, AI-driven automation, and scalable SaaS platforms to streamline regulatory workflows, strengthen quality and compliance systems, and support Industry 4.0 transformation across the healthcare ecosystem. By integrating regulatory expertise, R&D innovation, and digital transformation, we help our clients accelerate market access, reduce risk, and achieve sustainable growth in highly regulated global markets.
Regulatory Focus
NexHealth Synergy: Leading the Charge in Regulatory and Compliance Solutions.
At NexHealth Synergy LLC, a US-based global regulatory and healthcare consulting firm, we support medical device, pharmaceutical, and healthcare organizations in navigating complex and evolving international regulatory frameworks. Our expertise covers major global markets including the European Union, United States, United Kingdom, Canada, Japan, and emerging regulatory regions, enabling our clients to achieve efficient and compliant market access worldwide. We provide end-to-end regulatory services across the product lifecycle, from early regulatory strategy and classification to technical documentation, submissions, audits, and post-market compliance.
Our team specializes in key regulations such as EU MDR and IVDR, US FDA pathways including 510(k), De Novo, and PMA, UK MDR, Canadian Medical Device Regulations, and CDSCO requirements in India, along with global pharmaceutical regulatory frameworks. We work closely with manufacturers, startups, and healthcare innovators to ensure robust quality systems, risk management, clinical and performance evaluation, and ongoing regulatory support. By combining deep regulatory knowledge with digital tools and automation, we help organizations reduce approval timelines, strengthen compliance, and maintain sustainable global regulatory readiness.

Nano-formulation R&D Focus
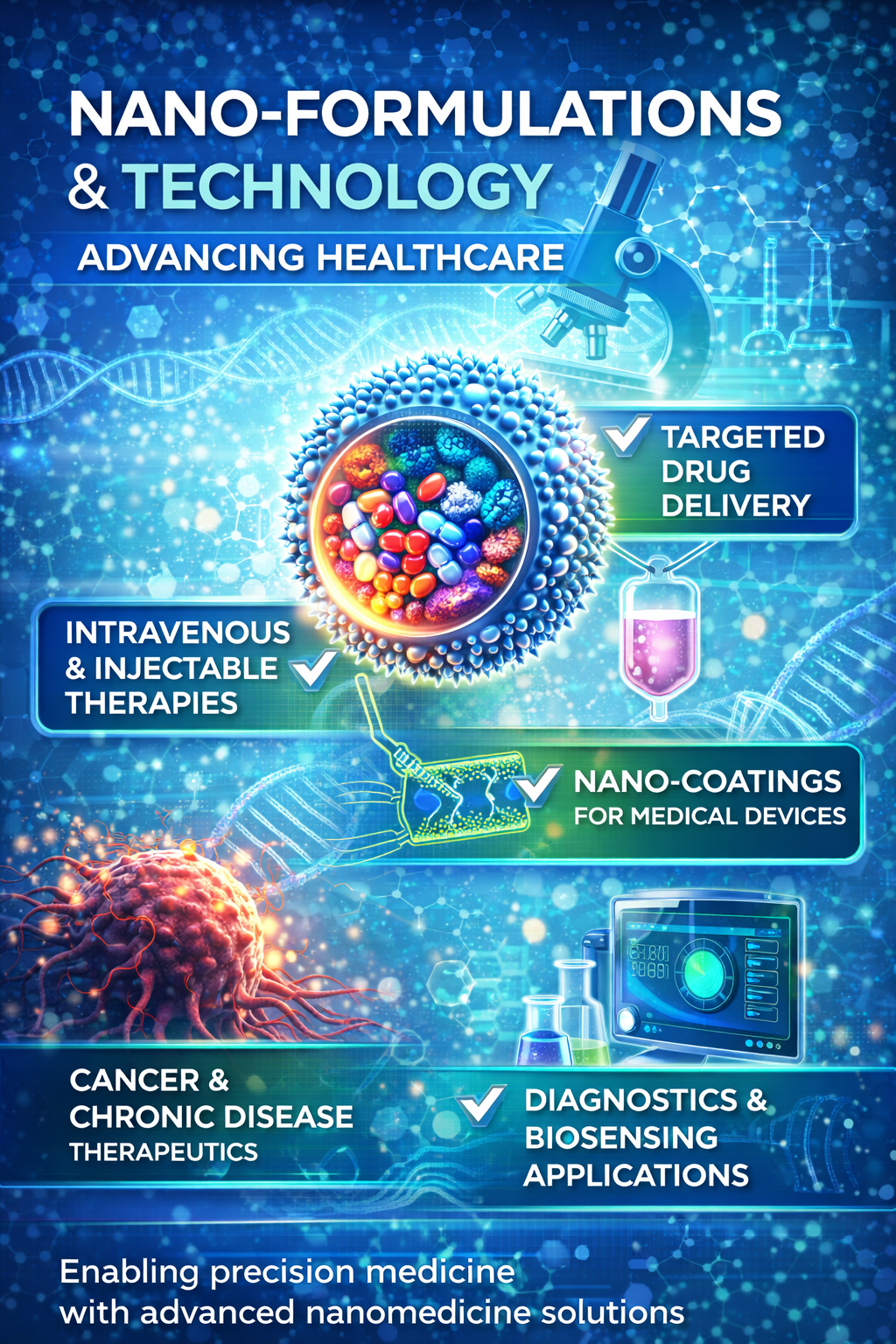
Advanced Nano-Formulation Innovation for Next-Generation Healthcare
- Targeted and controlled drug delivery systems for improved therapeutic outcomes
- Nano-enabled coatings and surface modifications for medical devices and implants
- Innovative nano-formulations for intravenous (IV) and injectable therapies
- Nanotechnology platforms for oncology, chronic diseases, and precision medicine
- Advanced nano-materials for diagnostics, imaging, and biosensing applications
- Scalable, regulatory-compliant development from concept to commercialization
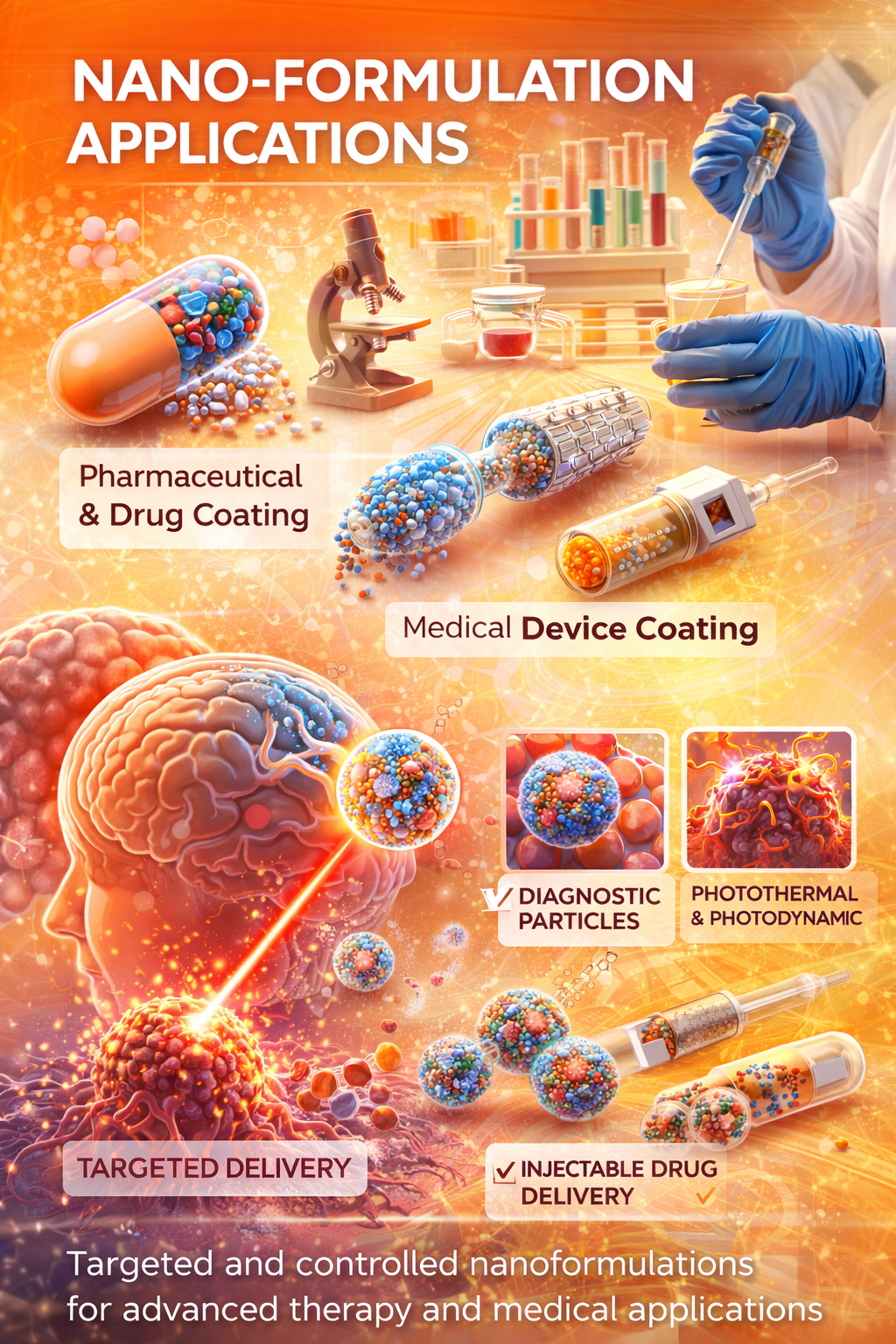
At NexHealth Synergy LLC, a US-based global healthcare innovation and regulatory consulting company, our nano-formulation R&D capabilities are focused on developing advanced, safe, and scalable solutions that address critical challenges in modern healthcare. We work across medical device, pharmaceutical, and diagnostic sectors to design next-generation nano-enabled products that enhance efficacy, safety, and patient outcomes. Our research integrates nanotechnology, material science, and translational healthcare innovation to support the development of targeted drug delivery systems, precision therapeutics, and smart medical solutions.
Our expertise includes the development of nano-formulations for intravenous and injectable therapies, nano-coatings for medical devices and implants, and nano-enabled platforms for pharmaceutical APIs and drug delivery. We also support innovation in oncology and chronic disease management through controlled and site-specific delivery approaches. In addition, we develop advanced nano-materials for diagnostics, imaging, and biosensing applications, enabling early detection and improved disease monitoring.
From early research and proof-of-concept to scale-up, technology transfer, and regulatory alignment, we provide end-to-end R&D support tailored to global compliance standards. By combining scientific excellence with regulatory expertise and digital innovation, we help healthcare organizations accelerate product development, reduce risks, and achieve successful commercialization in global markets.
Healthcare Digital Transformation, AI, and Industry 4.0 Solutions
Smart, Scalable, and Integrated Digital Platforms for Healthcare, Life Sciences, and Industrial Innovation
At NexHealth Synergy LLC, a US-based global healthcare and technology consulting company, we deliver AI-driven workflow automation, healthcare SaaS platforms, interoperability solutions, digital quality and compliance systems, Industry 4.0 smart manufacturing, real-time data analytics, connected device ecosystems, regulatory and clinical digitalization, cloud-based infrastructure, and secure system integration designed to enhance operational efficiency, compliance, scalability, and innovation across hospitals, diagnostics, pharmaceutical, medical device, and industrial healthcare sectors.
NexHealth Synergy LLC provides comprehensive digital transformation and software innovation services tailored for the global healthcare, pharmaceutical, medical device, and life sciences ecosystem. Our solutions enable organizations to transition from manual, fragmented workflows to intelligent, automated, and connected digital environments that support regulatory compliance, operational excellence, and data-driven decision-making. We design and deploy scalable web-based, mobile, and cloud-based SaaS platforms that streamline quality management, regulatory documentation, clinical and operational workflows, supply chain visibility, and compliance reporting across hospitals, diagnostic laboratories, and healthcare manufacturing facilities.
Our interoperability and integration capabilities ensure seamless connectivity across complex healthcare and industrial systems, including ERP, LIMS, HIS, EHR, MES, and connected medical devices. Through secure APIs, data exchange frameworks, and real-time monitoring platforms, we enhance traceability, transparency, and regulatory readiness across the value chain. We also support Industry 4.0 and smart manufacturing by implementing predictive maintenance, digital twins, intelligent process automation, and AI-driven quality control solutions for pharmaceutical, medical device, and industrial healthcare production environments.
In addition, our AI and analytics-driven platforms empower organizations with predictive insights, operational intelligence, and performance optimization. From regulatory forecasting and clinical data analytics to workflow automation and cybersecurity frameworks, NexHealth Synergy LLC delivers integrated digital solutions that improve efficiency, reduce risk, and accelerate innovation. By combining healthcare domain expertise, regulatory knowledge, and advanced technologies, we enable our clients to achieve scalable growth and sustainable competitive advantage in the evolving global healthcare landscape.

